

Cobalt is in the 7th column of the d block and therefore has 7 d electrons d7.

Cobalt is an inner transition metal which means the electron configuration will end in a d block. Cobalt is also in Group 9, so it must have 9 valence electrons. The s,p,d,f configuration for cobalt (Co) is 1s22s22p63s23p64s23d7, determined by the position of the element on the periodic table. 27 Cobalt Electron Configuration Group Garage 194 subscribers Subscribe No views 1 minute ago Group Garage quick video to walk through how and why to write the shorthand electron.
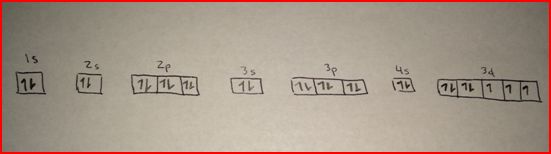
Co is in Period 4 of the Periodic Table, and Ar is the preceding noble gas. Exposure to cobalt-60, a powerful gamma ray emitter, may cause cancer. An electron diffraction and high-resolution electron microscopy study shows that oxygen vacancies in BaCoO 2. The electron configuration of Co3+ is Ar4s3d5. Brandt between 17 when he was able to show that cobalt colors glass a rich blue. This solid ferromagnetic silver-white element was known in ancient times for its compounds, but its discovery was credited to G. Cobalt should be handled with care because of its toxicity and its risk factor in nuclear confrontation. Frequently, cobalt is associated with nickel because both elements have characteristic ingredients of meteoric iron. In small amounts, cobalt is an essential element for humans and many other living organisms, and it is also a central component of vitamin B-12 or cobalamin. It does help you to just assume that's the case if you're writing an electron configuration but that's not what's happening in reality. But it's implying that the d orbitals, the 3d orbitals fill after the 4s orbital and is therefore a higher energy and that's not true actually. This solid ferromagnetic silver-white element was known in ancient times for its compounds, but its discovery was credited to G. That gives you the correct electron configuration, argon 4s 2, 3d 1. Exposure to cobalt-60, a powerful gamma ray emitter, may cause cancer.Obtained from: arsenic, oxygen, sulfur, cobatineįrequently, cobalt is associated with nickel because both elements have characteristic ingredients of meteoric iron. Cobalt should be handled with care because of its toxicity and its risk factor in nuclear confrontation. Co4+,Co3+,Co2+ and Co Electron Configuration Electron Configuration for Co, Co2+, and Co3+ (Cobalt and Cobalt Ions)This video has solved the following q. Brandt between 17 when he was able to show that cobalt colors glass a rich blue. Protons/Electrons: 27 Neutrons: 32 Shell structure: 2,8,15,2 Electron configuration: Ar3d74s2 Oxidation state: 2,3 Crystal structure: hexagonal. For example, to find the configuration for the lithium ion (Li), start with neutral lithium (1s2s). Introduction Electron configuration describes the distribution of electrons within an atom. Then, add or remove electrons depending on the ion's charge. Cobalt is in the first row of the d-block of elements. To find the electron configuration for an ion, first identify the configuration for the neutral atom. The electron configuration of cobalt is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 7 4s 2. This solid ferromagnetic silver-white element was known in ancient times for its compounds, but its discovery was credited to G. Answer and Explanation: Become a member to unlock this answer Create your account. Obtained from: arsenic, oxygen, sulfur, cobatineįrequently, cobalt is associated with nickel because both elements have characteristic ingredients of meteoric iron.


 0 kommentar(er)
0 kommentar(er)
